Project information
- Project Title: Clear Rays LED Skin Treatment Product
- Client: Quasar Biotec USA
- Project date: 01 March, 2012
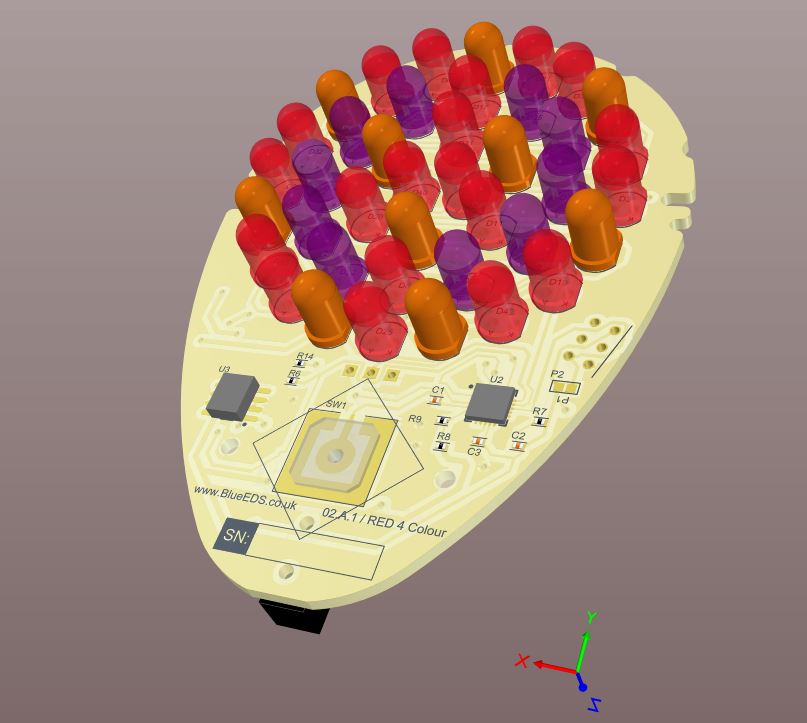
LED Skin Treatment Product:
For our longstanding American Client, based in Florida. A safe, FDA medically approved electronics control and drive electronics was specified by the client.
The product has 2 sides one side ultraviolet led’s and the other side Red led’s for treating skin conditions.
The led arrays needed to be timed for the specified user treatment and automatically turn off following the treatment time.
On completion of the treatment time the device would bleep to indicate the treatment was over. With a soft start and soft stop to allow the user to turn the device away from the eyes in the event of accidentally turning the device on while looking at it as this could be painful and possibly damage the eyesight.
The device would be powered from the mains with a simple wall power supply.
Many safety features were incorporated as required by FDA approval before release to market. Including auto shutdown in fault. Fail safe in the event of a firmware failure. Temperature and power monitoring plus many others were included as safety features.
The product is currently in worldwide distribution with a number of variants that have been developed with the client and included in the product range. eius.
Services supplied:
- Electronics design.
- PCB design.
- Mechanincal integration of electronics systems 3D CAD.
- Closed loop control.
- Mechanincal integration of electronics systems 3D CAD.
- FDA Submission support documentation.
- EMC Submission support documentation.
- Manufacturing Support